Why Does Carbon Have a High Melting Point
Well theoretically it does but it doesnt melt. Carbon atoms have four unpaired electrons and can form four covalent bonds.
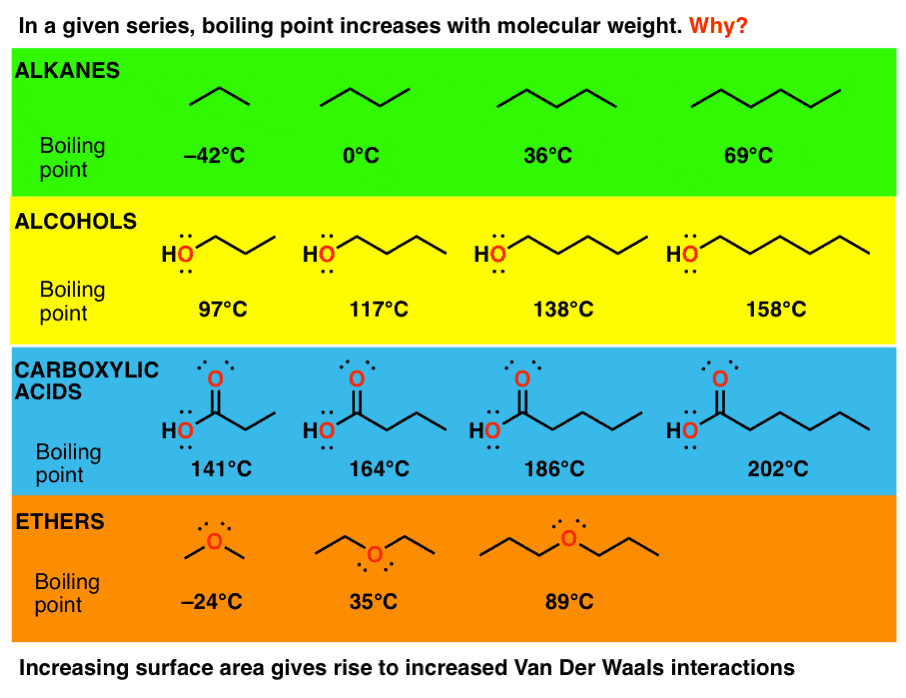
3 Trends That Affect Boiling Points Master Organic Chemistry
Like silica diamond has a very high melting point and it does not conduct electricity.

. It has the highest sublimation point of all elements. The melting point also defines a condition in which the solid and liquid can. Like diamond it has a high melting point.
In general melting is a phase change of a substance from the solid to the liquid phase. Well theoretically it does but it doesnt melt. The reason that tungsten is better then carbon for light filaments is because the melting point is higher.
Melting point of Carbon is 4099C. Hence graphite conducts electricity. It sublimes at around 3900 K.
It has the highest. Curium is a hard dense silvery metal with a relatively high. Carbon doesnt really have a melting point.
Why Does Carbon Have A High Melting Point. It happens in a curve. Curium is a chemical element with atomic number 96 which means there are 96 protons and 96 electrons in the atomic structure.
The melting point of carbon is. The chemical symbol for Curium is Cm. Each carbon is covalently bonded to three others so there is one electron delocalised per carbon atom which is free to move between the layers and carry charge.
It sublimes at around 3900 K. It has a high melting point and is a good conductor of electricity which makes it a suitable material for the electrodes. Why does diamond have a high melting point AQA.
This is the reason why diamond has a high melting point. Each carbon is covalently bonded to three others so there is one electron delocalised per carbon atom which is free to move between the layers and carry charge. This is because covalent bonds are strong.
Note that these points are associated with the standard atmospheric pressure. Hence graphite conducts electricity. Carbon atoms have four unpaired electrons and can form four covalent bonds.
Why does carbon have a high melting point Posted on March 24 2021 by admin Frequently asked questions. Each carbon atom is. So it will still be a solid even above its highest theoretical melting temperatu.
The rigid network of carbon atoms held together by strong covalent bonds makes diamond very hard. Answer 1 of 5. Carbon doesnt really have a melting point.
The melting point of a substance is the temperature at which this phase change occurs.

Carbon Structure Of Carbon Allotropes Britannica

Giant Covalent Macromolecules Secondary Science 4 All

New Material Has Higher Melting Point Than Any Known Substance Cmdnetwork

Boiling Point And Melting Point In Organic Chemistry Chemistry Steps
No comments for "Why Does Carbon Have a High Melting Point"
Post a Comment